Background: Fetal hemoglobin (HbF) is the most influential modifier of the clinical and hematologic phenotype of sickle cell disease (SCD) and is highly heritable. Low HbF is independently associated with increased white matter changes on brain imaging and poorer performance on neurocognitive measures (Ruffiuex, Child Neuropsychology, 2013). Our previous work has shown that 11 SNPs in three genes (BCL11A, MYB, and β-globin), contribute over 20% of the variance in HbF (Rampersaud, Kang. et al., 2020, under review). Clinically, these HbF-associated SNPs are associated with disease severity and frequency of pain events. However, the neurocognitive implications of these SNPs have yet to be explored. As part of a prospective longitudinal cohort study, the Sickle Cell Clinical Research and Intervention Program (SCCRIP), we evaluated the relationship between HbF-associated SNPs and neurocognitive functioning in SCD patients.
Methods: We included 257 patients with SCD (69% HbSS/HbSβ0-thalassemia). The median age of participants was 13 years (range 4 - 25). We extracted genotypes for the 11 HbF-associated SNPs from whole genome sequencing data and analyzed them based on an additive genetic model. Following informed consent, patients completed a battery of gold-standard neurocognitive measures, supervised by a psychologist, assessing IQ, verbal and perceptual reasoning, working memory, processing speed, sustained attention, and executive functioning skills. HbF was the average value of measurements collected within 3 months of neurocognitive assessment, or the closest value. In univariate analyses, Kruskal-Wallis rank sum test was used to assess the associations of the 11 HbF-associated SNPs with neurocognitive measures. Linear regression was used to examine these associations adjusting for age, sickle genotype, hydroxyurea exposure, socioeconomic status (index of social vulnerability) and 5 principal components to adjust for population stratification. Benjamini and Hochberg false discovery rate (FDR) was used for multiple corrections and FDR adjusted p (pFDR)<0.05 was considered significant for all analyses. Mediation analyses (Imai, Psychological Methods, 2010) were used to evaluate the indirect effects of HbF-associated SNPs on neurocognitive outcomes via HbF.
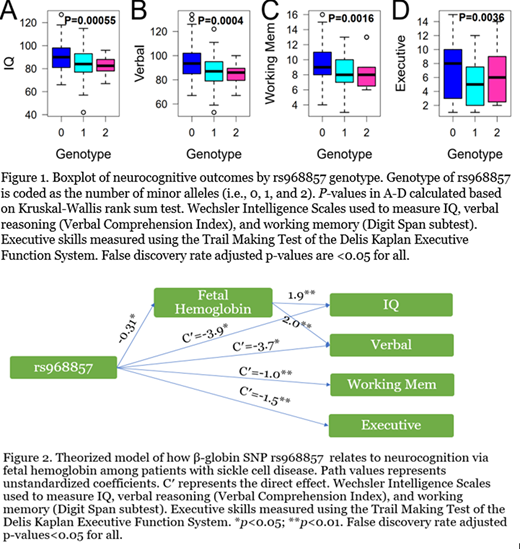
Results: One SNP in the β-globin gene, rs968857, was associated with performance on measures of IQ, verbal reasoning, working memory, and executive functioning by Kruskal-Wallis rank sum test (Figure 1) and by linear regression adjusting for covariates listed above at pFDR <0.05. HbF expression was positively associated with IQ and verbal reasoning scores at pFDR <0.05 using linear regression adjusting for age, sickle genotype, hydroxyurea exposure, and socioeconomic status. HbF mediated the relationship between rs968857 and scores on IQ and verbal reasoning measures at pFDR <0.05 (Figure 2). The direct effect of rs968857 on neurocognitive functioning remained significant following inclusion of HbF at pFDR <0.05, indicating partial mediation. Full linear regression models with rs968857, HbF, and covariates accounted for 10.8%, 14.5%, 6.4%, and 8.3% of the variance in IQ, verbal reasoning, working memory, and executive functioning measures, respectively.
Conclusions: This is the first study to demonstrate a relationship between genetic modifiers of SCD and neurocognitive functioning. Beyond findings on neuroimaging and sociodemographic factors, there is little known about risk for neurocognitive deficits in SCD. The present results suggest an influence of SNP rs968857 on cognitive function, which was partially mediated by expression of HbF. rs968857, is one of four SNPs that defines almost all β-globin gene cluster haplotypes and influences HbF levels in SCD. How this SNP effects HbF and neurocognition is unknown and requires further investigation of its mechanism. The highly heritable nature of HbF may allow for future use of precision medicine. SCD patients could be stratified according to risk for neurocognitive deficits to utilize early intervention strategies, informed by genetic polymorphisms. Correlation with neuroimaging will help further elucidate the relationship between genetic modifiers and neurocognitive functioning. Future trials with larger samples are needed to validate our findings and investigate if the observed relationships differ by age or hydroxyurea treatment status.
Estepp:Daiichi Sankyo, Esperion, Global Blood Therapeutics: Consultancy; Global Blood Therapeutics, Forma Therapeutics, Pfizer, Eli Lilly and Co: Research Funding; ASH, NHLBI: Research Funding. King:Amphivena Therapeutics: Research Funding; Bioline: Consultancy; Celgene: Consultancy; Cell Works: Consultancy; Incyte: Consultancy; Magenta Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novimmune: Research Funding; RiverVest: Consultancy; Tioma Therapuetics: Consultancy; WUGEN: Current equity holder in private company. Hankins:Novartis: Research Funding; National Heart, Lung, and Blood Institute: Honoraria, Research Funding; UptoDate: Consultancy; Global Blood Therapeutics: Consultancy, Research Funding; MJH Life Sciences: Consultancy, Patents & Royalties; American Society of Pediatric Hematology/Oncology: Honoraria; LINKS Incorporate Foundation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal